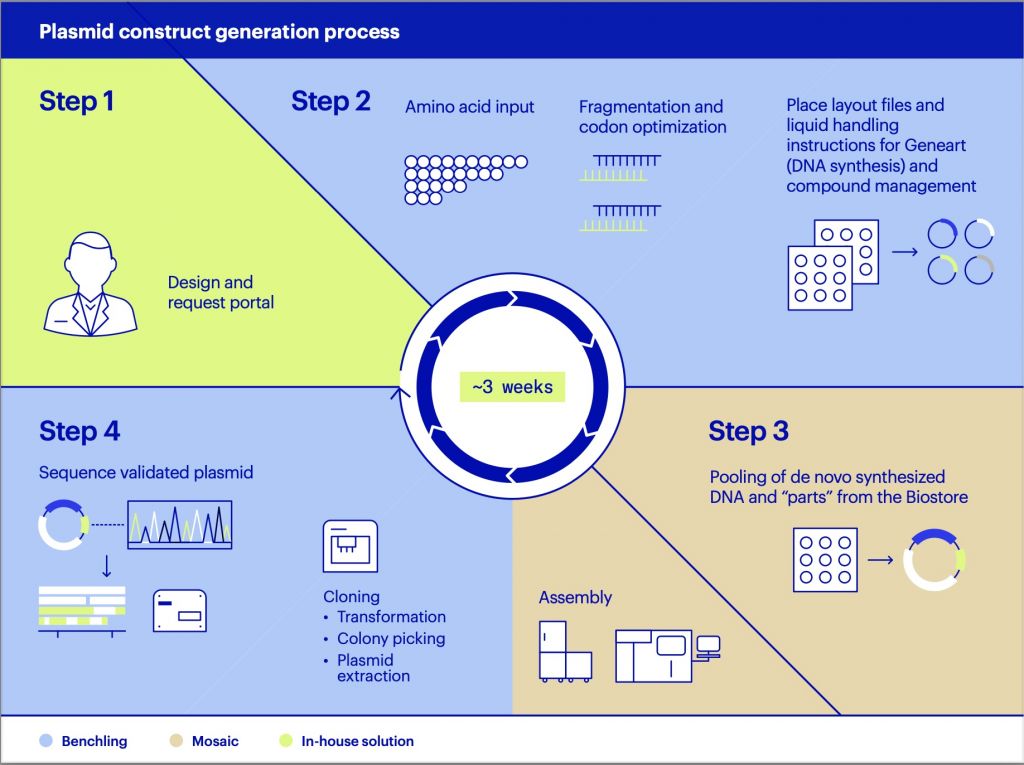
IMAGE: Zα mutations from one parental chromosome map directly to phenotype as there is no transcript from the other parental chromosome to cover up defects in the ADAR protein produced. view more
Credit: Alan Herbert
DNA has many different forms. Normally the two strands of DNA wind around each other in a right-handed spiral. However, another conformation, called Z-DNA, exists where the strands twist to the left. The function of the Z-DNA has remained a mystery since its discovery. A newly published paper unambiguously establishes that the Z-conformation is key to regulating interferon responses involved in fighting viruses and cancer. The study analyzes families with variants in the Z-binding domain of the ADAR gene.
The peer-reviewed results published online in the European Journal of Human Genetics end the long-standing debate as to whether the unusual left-handed Z-conformation has any biological function. Z-DNA forms when right-handed B-DNA is unwound to make RNA. An analysis of genetic mutations in Mendelian families by Alan Herbert at InsideOutBio reveals that the Z-conformation regulates those type I interferon responses normally induced by viruses and tumors. The study confirms a biological role for the left-handed conformation in human disease and reveals that the human genome encodes genetic information using both shape and sequence. The two codes are overlapping, with three-dimensional shapes like Z-DNA and Z-RNA forming dynamically, altering the read-out of sequence information from linear, one-dimensional DNA chromosomal arrays.
One approach to understanding the biological role of Z-DNA has been to isolate proteins that bind specifically to the left-handed Z-DNA conformation and study their function. Alan Herbert and the late Alexander Rich led a team at MIT that identified the Zα domain, which binds very tightly to both Z-DNA and Z-RNA. X-ray studies revealed that the binding was specific for the Z-conformation without any sequence specificity. The co-crystals of Zα and Z-DNA allowed identification of key protein residues in their interaction.
The Zα domain is present in a double-stranded RNA editing enzyme called ADAR. ADAR edits double-stranded RNAs (dsRNA) that usually form when an RNA transcript basepairs with itself. The enzyme changes adenosine to inosine, which is then readout as guanosine, changing both the information of the RNA and its downstream processing, generating many different RNA products from a single transcript. Early studies suggested ADAR was involved in anti-viral interferon responses. However, most edited dsRNA in a cell originate from repetitive Alu elements, fragments of non-coding RNA that colonized the human genome early in its evolution through a process of copy and paste. Recent studies show that suppression of such dsRNAs by ADAR editing is vital to the survival of many tumors.
The discovery of families with mutations in the ADAR gene has now revealed a biological function for the left-handed conformation. Families with loss of function ADAR variants over-produce interferon, leading to a severe diseases such as Aicardi-Goutières syndrome (OMIM: 615010) and Bilateral Striatal Necrosis/Dystonia. In some families, due to the different ADAR variants inherited from each parent, only one parental chromosome is capable of making ADAR protein. In such families, it is possible to map a mutation directly to phenotype. Individuals with Zα ADAR variants that no longer bind the Z-conformation have impaired dsRNA editing and exaggerated dsRNA induced interferon responses, confirming that the left-handed Z-conformation regulates these responses. The findings directly link Z-DNA to human disease and unambiguously establish a biological role for this alternative nucleic acid conformation.
The switch in shape from right-handed to left-handed DNA alters the readout from genes involved in the type I interferon pathway. Only a subset of sequences flip to form Z-DNA under physiological conditions. Their distribution within the genome is non-random. These flipons create phenotypic diversity by altering how genes generate RNA. They are subject to selection just like any other variation. The genomes that emerge encode genetic information in both shape and sequence with frequent overlap between the two different instruction sets.
###
Dr. Herbert leads discovery at InsideOutBio, a startup working on complement pathways to enhance immune responses against tumor antigens. Additional information on the role of the left-handed conformation in human disease is available here:
Z-DNA and Z-RNA in human disease (https:/ / doi. org/ 10. 1038/ s42003-018-0237-x)
ADAR and Immune Silencing in Cancer
(https:/ / doi. org/ 10. 1016/ j. trecan. 2019. 03. 004)