Using a technique called “DNA origami,” researchers created traps that encase large viruses—such as SARS-CoV-2, influenza A, and Zika—in hopes of preventing them from infecting cells.
A study published today (January 18) in Cell Reports Physical Science details how researchers used DNA origami to engineer strands of genetic material into Lego-like structures that form a cage around large pathogens. While the study only looked at how effectively the structures bound to viruses in vitro, the traps could one day help clear viruses from the body.
“It’s a fantastic paper,” says Ashwin Gopinath, a biomechanical engineer at MIT who was not involved in the study. “It’s a really interesting physical approach to virus entrapment.”
Study coauthor Hendrick Dietz, a physicist at the Technical University of Munich, hopes that these viral traps could one day be used to treat any virus. “There are more than 200 known viruses. For only five percent of those, we have actual medication that you can use to treat an acute infection,” he says.
DNA is a super flexible and modular structure, meaning that with the right sequences, scientists can use it to create a vast array of structures of different shapes and sizes. The term DNA origami was first coined more than a decade ago to describe a method of forming 3D shapes with DNA and has more recently been used to design methods of trapping viruses with antibody-lined shells before they reach their host cells. In previous work, Dietz and his colleagues used DNA origami techniques to engineer structures that could envelop small viral particles less than 85 nm in diameter, preventing them from inserting their DNA into their hosts. But since many common and devastating viruses, such as influenza A, Zika, and coronaviruses, are larger than 100 nm in diameter and come in several shapes, from peanut-shaped to filamentous, Dietz says that he and his team wanted to try to make these cages bigger and more modular.
To create their viral traps, the team started with a single-stranded DNA molecule that was either synthesized or derived from a bacteriophage virus. They mixed this DNA with shorter, synthetic DNA strands designed to adhere to specific sequences of the larger single-stranded DNA sequence. The shorter strands provided instructions for the large molecule to twist and fold, forcing it to hold a desired shape.
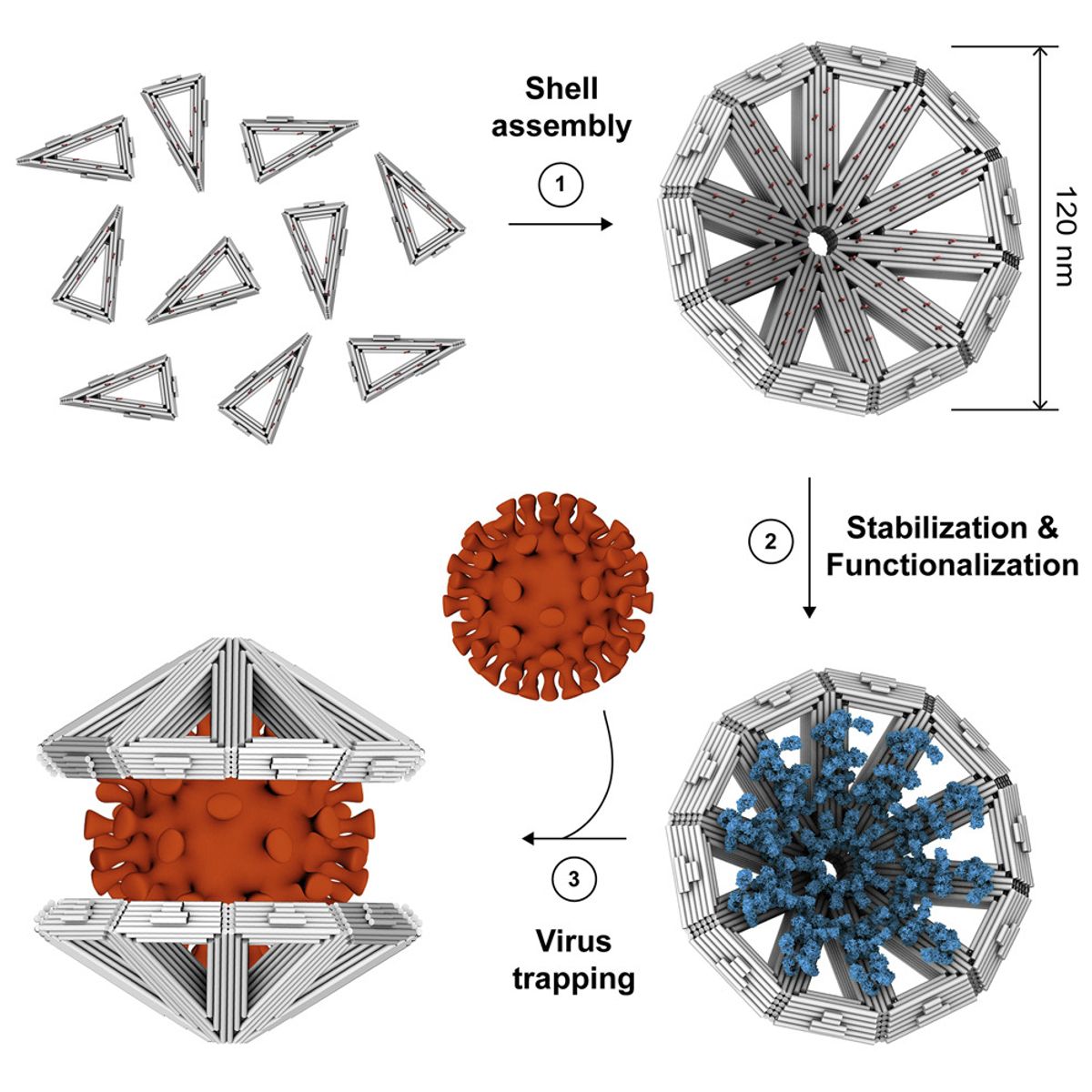
Using DNA origami, the team designed 2D triangle-shaped building blocks that snap together, edge to edge, like puzzle pieces. Then, using cryo-electron microscopy (cryoEM), the researchers confirmed that the triangles assembled themselves into cone-shaped, multisided shells. The team then coated the inside of each shell with virus-binding substances such as antibodies. These shells can sandwich themselves together around viruses, encasing a viral particle more than 100 nm in diameter, which could, in theory, cordon the virus off from a potential host cell and prevent infection, though the team didn’t test for clinical outcomes. Importantly, the shells could also be coated with other virus-binding substances. In this case, the researchers used heparan sulfate, a substance that sticks to many viral protein coats.

Researchers crafted triangle-shaped building blocks made of DNA. These building blocks could (1) combine to form cone-shaped shells. After stabilizing (2) these shells to survive physiological conditions, the researchers showed they could trap large viruses (3), including SARS-CoV-2 and influenza.
Adapted from Monferrer et al., DNA origami traps for large viruses, Cell Reports Physical Science (2022), https://doi.org/10.1016/j.xcrp.2022.101237
The researchers tested the cones’ ability to assemble around four viruses—influenza A, Zika, chikungunya, and SARS-CoV-2—by mixing them with virus-like particles, small particles that contain proteins from the virus’s external protein coat. They used cryoEM to confirm that the shells, each of which had been coated with either heparin sulfate or antibodies specific to one of the viruses, had bound to all of the viruses effectively.
One hurdle in the assembly process was the finding that the shells, assembled in solutions with high salinity, fell apart under physiological conditions, especially when exposed to low salinity. So, to stabilize the assembled cones further, the researchers used UV light to strengthen the bonds between the building blocks, which prevented the shells from degrading at the relatively low salt concentrations found in the body. They also covered the assembled structures with an oligosine polymer-based coating, preventing them from being degraded by nucleases. On the whole, the process was faster and more efficient than existing DNA origami-based virus-capture techniques, which use multiple types of building blocks, Dietz says.
According to Dietz, DNA origami traps may be superior to antibody treatments, which are often used to treat viral infections, because “[o]ur shells don’t accumulate mutations over time,” he explains, adding that the shells could also be coated with a more general virus-targeting substance, such as heparin sulfate, perhaps eliminating the need for antibodies entirely.
“From a technical point of view, it’s a really monumental effort,” Gopinath says, but he adds that it’s still unclear whether such complex structures will be necessary to effectively trap large viruses. Simpler techniques that use fewer subunits or molecules might be just as effective, he says.
He also adds that while similar techniques have been found not to cause an immune response, adding such a large number of these molecules into the body could potentially cause problems. In any case, “in vivo experiments in mice are the next step,” he says.














