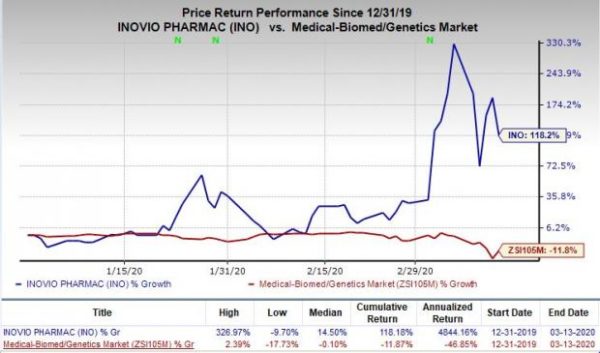
Shares of Inovio Pharmaceuticals, Inc. have surged significantly in the year so far. The stock has skyrocketed 118.2% against the industry’s decline of 11.8%.

In January 2020, the company received a grant of up to $9 million from Coalition for Epidemic Preparedness Innovations (“CEPI”), a public-private non-profit organization, for the pre-clinical and phase I development of INO-4800, its DNA vaccine, to treat COVID-19, the disease caused by the novel coronavirus. Currently, INO-4800 is in preclinical testing, designed to precisely match the DNA sequence of the deadly virus.
The company expects to begin human clinical studies on INO-4800 in April. Clinical studies in China and South Korea are expected to begin shortly, thereafter. Shares of the company soared significantly on this news.
Importantly, by 2020-end, Inovio plans to deliver one million doses of INO-4800 for further clinical studies and emergency use to treat the coronavirus-infected people. If successfully developed, this can be a huge boost for the company as the outbreak of COVID-19 has been a disaster for mankind.
Meanwhile, earlier this month, Inovio received a new $5-million grant from the Bill & Melinda Gates Foundation to accelerate the testing and scale up its proprietary CELLECTRA 3PSP smart device for the intradermal delivery of INO-4800. The company claims that the smart delivery device will be able to support large scale manufacturing of INO-4800 doses by the end of 2020.
Though the coronavirus vaccine program is currently driving Inovio’s stock, its other vaccine candidates also hold potential.
We remind investors that, VGX-3100, a human papillomavirus (HPV) immunotherapy, is the most advanced candidate in Inovio’s pipeline right now.
VGX-3100 is currently being evaluated in a phase III study (REVEAL 1) for the treatment of cervical dysplasia caused by papillomavirus (HPV). Top-line efficacy data from the REVEAL 1 program are expected by the fourth quarter of 2020.
VGX-3100 is also being evaluated in two phase II studies for the treatment of anal dysplasia and vulvar dysplasia caused by HPV.
Meanwhile, in February 2020, the FDA accepted Inovio’s investigational new drug (IND) application for its novel DNA medicine, INO-3107. The regulatory body’s acceptance allows the company to begin a phase I/II study to evaluate the candidate for the treatment of patients with recurrent respiratory papillomatosis (RRP), a rare disease caused by certain types of HPV.
Notably, last November, Inovio announced positive interim data from the phase II study on its immuno-oncology combo of INO-5401 and INO-9012 in combination with Regeneron (REGN – Free Report) and Sanofi’s (SNY – Free Report) PD-1 inhibitor, Libtayo (cemiplimab), for addressing newly-diagnosed patients with glioblastoma (GBM). The company plans to present 12-month overall survival data from the same in the next quarter.
Apart from this, Inovio is working on developing vaccines for Ebola, Zika, Lassa Fever and the Middle East respiratory syndrome (MERS) virus.
Any positive development of these pipeline candidates, mainly INO-4800 and VGX-3100, should be a major boost for the stock in 2020.
Inovio Pharmaceuticals, Inc. Price
Zacks Rank & A Key Pick
Inovio currently carries a Zacks Rank #3 (Hold). Another top-ranked stock in the biotech sector is Ovid Therapeutics Inc. (OVID – Free Report) , which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Ovid Therapeutics’ loss per share estimates have been narrowed 29.8% for 2020 over the past 60 days. The stock has rallied 15.4% in the past year.
Today’s Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2019, while the S&P 500 gained and impressive +53.6%, five of our strategies returned +65.8%, +97.1%, +118.0%, +175.7% and even +186.7%.
This outperformance has not just been a recent phenomenon. From 2000 – 2019, while the S&P averaged +6.0% per year, our top strategies averaged up to +54.7% per year.