- Research
- Open Access
- Published:
Parasites & Vectors volume 16, Article number: 4 (2023)
Abstract
Background
Trypanosoma evansi infects a large number of wild and domestic animals and causes a spoliative disease known as surra. It is mechanically transmitted, mainly by biting flies of the genera Tabanus and Stomoxys. The detection of T. evansi DNA in the feeding apparatus of Dichelacera alcicornis and Dichelacera januarii from South America is reported, to the best of our knowledge, for the first time.
Methods
Tabanids were collected weekly from February 2018 to February 2019 from two sites. The feeding apparatus was removed and DNA extraction, polymerase chain reaction and sequencing were performed.
Results
A 205-base pair fragment of the variant surface protein RoTat 1.2 gene, confirmed by DNA sequencing, was amplified from the feeding apparatus of D. alcicornis and D. januarii.
Conclusions
This is, to the best of our knowledge, the first record of T. evansi DNA in South American tabanids.
Graphical abstract

Background
The family Tabanidae, which belongs to the order Diptera, comprises approximately 4400 species [1]. These insects are of great medical and veterinary interest due to their persistent and irritating bites and their ability to transmit 32 pathogens, including viruses, bacteria, helminths, and protozoa, such as Trypanosoma spp. [1].
Trypanosoma evansi (Steel, 1885) is the etiological agent of surra, a disease that affects a variety of domestic and wild mammals. Trypanosoma evansi is distributed in Africa, Asia, and South America and causes important economic losses to livestock farming. Surra is of great concern because of the absence of pathognomonic signs, including fever, anemia, loss of body weight, low production of animal protein, nervous signs, abortion, cachexia, and death, with or without more particular signs related to the host species [2]. Trypanosoma evansi, which is mechanically transmitted by tabanids and is widespread in regions of Brazil and the rest of South America, causes outbreaks of surra in horses [3].
The focus of most of the published literature on tabanid species from Brazil and the rest of South America is their taxonomy [4,5,6,7], ecology [8, 9] and behavior [10, 11] in different biomes, and molecular tools were not used for identification or bioprospecting in these studies. More specifically, few studies have addressed tabanids in southern Brazil [12,13,14,15,16,17], and only one reported the molecular detection of a strain of a trypanosomatid, Trypanosoma kaiowa, which is associated with the crocodilian clade of Trypanosoma [18]. Cases of trypanosomosis caused by T. evansi [19] in that region have been described in which the involvement of tabanids was assumed. There are no data on the detection of T. evansi in tabanid flies in South America. This is, to the best of our knowledge, the first study to describe the molecular detection of T. evansi DNA in Dichelacera alcicornis (Wiedemann, 1828) and Dichelacera januarii (Wiedemann, 1819), both of which are widely distributed in various biomes of South America [20] and may be related to outbreaks of surra.
Methods
Study site
This study was carried out in the municipality of Lages (27°48′57″S, 50°19′33″ W), Santa Catarina State, Brazil, which has a mixed rainforest terrain and altitude of 930 m above mean sea level.
The Köppen climate classification of the municipality is Cfb, i.e. it has a temperate oceanic climate. The average temperature in the coldest month is above 0 °C, the average annual temperature is below 22 °C, and at least 4 months have an average temperature above 10 °C, with no significant difference in precipitation between the seasons [21].
Sample collection
Tabanids were collected weekly from February 2018 to February 2019 from two rural properties located in different parts of the region in which especially cattle and horses are reared.
The collections were made once a week at the same location on each property, close to lakes and rivers, for 3 h, from 3 p.m. until 6 p.m., using a contained horse from each property as bait throughout the collection period. Tabanids that landed on the horses were carefully captured with a glass tube (4.5 cm diameter, 9 cm length) [14]. The flies were placed in individual plastic bottles using a SECTAB device [22]. The collected insects were transported to the Laboratory of Hemoparasites and Vectors (Lages, Santa Catarina, Brazil) and killed with chloroform in flasks.
Taxonomic identification of tabanids
Taxonomic identification was performed according to the taxonomic keys described by Fairchild and Philip [23], and Dr. Inocêncio de Sousa Gorayeb (Museu Paraense Emílio Goeldi, Belém, Brazil) verified the identification of the species. The collected specimens were deposited at the Entomology Museum of the Federal University of Fronteira Sul, Laranjeiras do Sul campus, Paraná, Brazil.
DNA extraction
The extraction of DNA from the mouthparts was initially carried out for the three most abundant species of tabanid. After these analyses, DNA was also extracted from the other species of Dichelacera captured, D. januarii.
The flies were washed twice with 70% ethanol solution and twice with sterile distilled water. The mouthparts were removed with the aid of sterile fine scissors under an entomological magnifying glass. The mouthparts were stored in 1.5-mL microtubes in Tris–NaCl–ethylenediaminetetraacetic acid (ETDA) buffer (10 mM Tris base, 200 mM NaCl and 50 mM EDTA), and stored frozen at -80 °C until DNA extraction.
For DNA extraction, the mouthparts were placed in a 1.5-mL microtube and macerated with an appropriate sized pistil. The DNA sample was extracted once with phenol (pH 7.8), once with phenol:chloroform (1:1), and once with chloroform:isoamyl alcohol (24:1). The DNA of the sample was then precipitated using sodium acetate (pH 6.0) and absolute ethanol and resuspended in 50 µL Tris–EDTA buffer (10 mM Tris–HCl, 1 mM EDTA) [24].
Polymerase chain reaction amplification
Flies were individually screened for the presence of Trypanosoma evansi and Trypanosoma vivax using specific oligonucleotide primers, RoTat 1.2 forward 5′GCGGGGTGTTTAAAGCAATA3′ and RoTat 1.2 reverse 5′ATTAGTGCTGCGTGTGTTCG3′ for T. evansi [24], and TviSL1 5′GCTCTCCAATCTTAACCCTA3′ and TviSL2 5′GTTCCAGGCGTGCAAACGTC3′ for T. vivax [25]. The first two primers amplified a 205-base pair (bp) fragment and the second two a 210-bp fragment. Polymerase chain reaction (PCR) was conducted in a 400-µL reaction mixture comprising 323.5 µL deionized water, 10.5 µL MgCl2, 1.75 µL Taq DNA polymerase, 7 µL deoxyribonucleotide triphosphate mix (10 mM), 35 µL of 10× Taq DNA polymerase buffer, 10.5 µL of each forward primer, 7 µL of each reverse primer, and 5 µL of the template. Trypanosoma evansi DNA was obtained from parasites purified from the blood of experimentally infected albino rats. Trypanosoma vivax DNA was obtained from purified parasites of experimentally infected sheep (approved by the Animal Experimentation Ethics Committee of Universidade do Estado de Santa Catarina) and was used as a positive control. Nuclease-free water was added to the PCR mix instead of a DNA sample as a negative control.
PCR was performed using an automated DNA thermal cycler (Biocycler). The amplification conditions were: initial denaturation at 94 °C for 3 min followed by 35 denaturation cycles at 94 °C for 30 s, annealing at 62 °C for 30 s, primer extension at 72 °C for 1 min, and a final extension at 72 °C for 4 min. The final phase of the PCR included cooling the samples to 10 °C. The PCR products were visualized on a 1% agarose gel stained with ethidium bromide.
Sequencing and Basic Local Alignment Search Tool
The PCR amplicons were purified using the QIAGEN Gel Purification Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. Sequencing was conducted using the BigDye Terminator Cycle Sequencing Kit according to the manufacturer’s protocol (Applied Biosystems, Carlsbad, CA). The eluent was loaded into a 96-well plate which was placed into an ABI Prism 3500 Genetic Analyzer (Applied Biosystems).
Each DNA sample was purified according to the following protocol: 50 µL sample DNA was added to a mixture containing 5 µL of 3 M sodium acetate, 125 µL of 100% ethanol, and 2 µL glycogen (20 mg/ml) and placed in a freezer at − 80 °C for 1 h. Following centrifugation at 12,000 g for 45 min at 4 °C, the pellet formed was washed once with 75% ethanol and centrifuged for another 15 min at 75,000 g at 4 °C. The mixture was then dried in a SpeedVac at 20–25 °C for 30 min and resuspended in 20 µL ultrapure water (Milli-Q).
The retrieved gene sequences were edited using BioEdit software [26]. The nucleotide Basic Local Alignment Search Tool (BLASTn) was used (www.ncbi.nlm.nih.gov/blast/) to confirm the sequences obtained from the PCR analysis. Gene sequences with match scores of 80–100% similarity were considered significant.
Results
A total of 523 female tabanids were collected from February 2018 to February 2019, specifically in February, March, April, November and December 2018, and January and February 2019. There was no evidence of tabanids in the other months of the collection period.
Individuals of 14 species and seven genera were collected (Table 1). Dichelacera alcicornis was the most abundant species, representing 52.77% (276) of the total, followed by Chrysops fusciapex (Lutz, 1909a) (17.97%, 94) and Chrysops patricia (Pechuman, 1953) (10.70%, 56), similar to the results of previous studies [11, 14]. The least abundant species were Tabanus fuscus (Wiedemann, 1819), Tabanus nebulosus (De Geer, 1776) and Acanthocera kroeberi (Fairchild, 1939), and represented 0.19% of the total.
Samples of the three most abundant species, D. alcicornis (Additional file 1: Fig. 1), C. patricia and C. fusciapex, were analyzed. Samples of D. januarii (Additional file 2: Fig. 2) were also examined so that both species of Dichelacera that had been captured were included in the analyses. The PCR amplicons were positive for T. evansi using the 205-bp RoTat 1.2 gene. Sequencing and analysis of the amplicons demonstrated that the sequence obtained corresponded to the T. evansi variable surface glycoprotein (accession no. MZ209177.1). It was present in the feeding parts of D. alcicornis (Da1) with 96% identity at site 1 and in D. alcicornis and D. januarii (Da 2) with 99% identity at site 2 (Fig. 1). There was no amplification of the T. vivax splice leader gene for any of the samples. The sequences were deposited in GenBank (respective accession numbers OM971942 and OM971943).
Multiple sequence alignment between the polymerase chain reaction amplified sequences of DNA from the feeding apparatus of Dichelacera alcicornis (Da1) and Dichelacera januarii (Da2) and the variant surface glycoprotein sequence of T. evansi deposited in GenBank
For C. patricia and C. fusciapex there was no amplification of the T. evansi RoTat 1.2 gene or T. vivax TviSL gene. The presence of DNA of other trypanosomatids was not evaluated due to the focal objective of the work.
Discussion
The few studies that exist on the seasonality of the Tabanidae in southern Brazil show D. alcicornis to be one of the most abundant species of this family in the region [6, 9, 10], as also found in the present study. The circulation of T. evansi in southern Brazil has already been demonstrated in several studies [14, 27,28,29], but this is the first one, to the best of our knowledge, in which the DNA of this parasite has been detected in the mouthparts of members of the Tabanidae. The sequenced PCR products showed high identity with the deposited sequences in GenBank, which is considered evidence of the presence of T. evansi DNA in the mouthparts of the tabanids examined here.
Two recent studies carried out in Brazil (in the Pantanal region and on the Coastal Plain of the state of Rio Grande do Sul) identified Trypanosoma kaiowa in tabanids using an insect dissection method and the observation of internal organs under an optical microscope [30] and through molecular detection [18]. Protozoan parasites have been detected in tabanids from other continents, such as Africa (particularly South Africa and Zambia), and included Trypanosoma congolense, Trypanosoma theileri, and Trypanosoma evansi [31]. In Europe (Poland), four species of tabanids (Haematopota pluvialis, Tabanus bromius, Tabanus maculicornis, and Tabanus distinguendus) were found to be infected by trypanosomatids of the subgenus Megatrypanum, of which Trypanosoma theileri is a member, and the occurrence of a trypanosomatid in T. maculicornis and T. distinguendus was described in that study for the first time [32]. Chrysops laetus and Dichelacera tetradelta infected with T. theileri were recently described for northern Brazil [33]. Our data, are, to the best of our knowledge, the first molecular confirmation of the presence of T. evansi DNA in the feeding apparatus of D. alcicornis and D. januarii, and support previous findings.
Although reservoirs play a central role in the maintenance and expansion of T. evansi infections, transmission depends on several ecological and epidemiological characteristics, such as the presence of competent vector species and susceptible mammalian hosts. To the best of our knowledge, there have been no studies on the vectorial capacity of the genera and species examined in the present study. However, a study has demonstrated the transmission of T. evansi by species of the genus Stomoxys under laboratory conditions [34]. A mathematical model has been developed that demonstrates the conditions necessary for the successful mechanical transmission of pathogens by tabanids [35]. Thus, the development and use of molecular detection approaches could help improve the identification of disease-causing agents and their tabanid vectors, in addition to facilitating the mapping of the circulation of these agents.
Conclusion
This is, to the best of our knowledge, the first report of T. evansi DNA in South American tabanids.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PCR:
-
Polymerase chain reaction
- EDTA:
-
Ethylenediaminetetraacetic acid
References
-
Baldacchino F, Desquesnes M, Mihok S, Foil LD, Duvallet G, Jittapalapong G. Tabanids: neglected subjects of research, but important vectors of disease agents. Infect Gen Evol. 2014;28:596–615. https://doi.org/10.1016/j.meegid.2014.03.029.
-
Desquesnes M, Holzmuller P, Lai DH, Dargantes A, Lun ZR, Jittaplapong S. Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed Res Int. 2013. https://doi.org/10.1155/2013/194176.
-
Büscher P, Gonzatti MI, Hébert L, Inoue N, Pascucci I, Schnaufer A, et al. Equine trypanosomosis: enigmas and diagnostic challenges. Parasite Vectors. 2019;12:234. https://doi.org/10.1186/s13071-019-3484-x.
-
Henriques Al, Krolow TK, Zaarchi TBO, Camargo LMA. Description of Tabanus rondoniensis (Diptera: Tabanidae), a new species of horsefly from the state of Rondônia, Brazil. Biodivers Data J. 2022;10:e76904. https://doi.org/10.3897/BDJ.10.e76904.
-
Lima HIL, Krolow TK, Henriques AL. A new species of Dichelacera (Dichelacera) Macquart (Diptera, Tabanidae) from the Brazilian savannah. Neotrop Entomol. 2018;47:380–4. https://doi.org/10.1007/s13744-017-0568-1.
-
Guimarães RR, Gorayeb IS, Rodrigues-Guimarães R, Seppa GS, Carvalho RW. Description of Dichelacera (Dichelacera) walteri n. sp. (Diptera: Tabanidae) with a key to related species in the subgenus Dichelacera Macquart. Neotrop Entomol. 2015;44:474–80. https://doi.org/10.1007/s13744-015-0311-8.
-
Krolow TK, Lucas M, Henriques AL. Revisiting the tabanid fauna (Diptera: Tabanidae) of Uruguay: notes on the species of the genus Tabanus Linnaeus, with the description of a new species. Neotrop Entomol. 2022;51:447–57. https://doi.org/10.1007/s13744-022-00958-7.
-
Yamazaki A, Suganuma K, Kayano M, Acosta TJ, Saitoh T, Valinotti MFR, et al. Risk factors for equine trypanosomosis and hematological analysis of horses in Paraguay. Acta Trop. 2022;233:106543. https://doi.org/10.1016/j.actatropica.2022.106543.
-
Ferreira RLM, Henriques AL, Rafael JA. Activity of tabanids (Insecta: Diptera: Tabanidae) attacking the reptiles Caiman crocodilus (Linn.) (Alligatoridae) and Eunectes murinus (Linn.) (Boidae), in the central Amazon, Brazil. Mem Inst Oswaldo Cruz. 2002;97:133–6. https://doi.org/10.1590/S0074-02762002000100024.
-
Barros ATM. Seasonality and relative abundance of Tabanidae (Diptera) captured on horses in the Pantanal, Brazil. Mem Inst Oswaldo Cruz. 2001;96:917–23. https://doi.org/10.1590/S0074-02762001000700006.
-
Bassi RMA, Cunha MCI, Coscaron S. A study of behavior of tabanids (Diptera, Tabanidae) from Brazil. Acta Biol Par. 2000;29:101–15. https://doi.org/10.5380/abpr.v29i0.585.
-
Krolow TK, Krüger RF, Ribeiro PB. Illustrated key for Tabanidae (Insecta: Diptera) genera of Campos Sulinos biome, Rio Grande do Sul. Brazil Bio Neotrop. 2007;7:253–64.
-
Turcatel M, Carvalho CJB, Rafael JA. Horseflies (Diptera: Tabanidae) of Paraná state, Brazil: pictorial identification key for subfamilies, tribes and genera. Bio Neotrop. 2007;7:265–78. https://doi.org/10.1590/S1676-06032007000200029.
-
Miletti LC, Colombo BB, Cardoso CP, Stalliviere FM, Tavares KCS, Komati LKO, et al. Prevalence, seasonality, and behavior of Tabanidae (Diptera) captured on a horse in the Planalto Serrano of Santa Catarina State, Brazil. Int J Trop Insect Sci. 2011;31:122–6. https://doi.org/10.1017/S1742758411000130.
-
Dutra RRC, Marinoni RC. Insetos capturados com armadilha malaise na Ilha do Mel, Baia de Paranágua, Paraná, Brasil. II. Tabanidae (Diptera). Rev Bras Zool. 1994;11:247–56. https://doi.org/10.1590/S0101-81751994000200007.
-
Bassi RMA. Descrição de Fidena campolarguense sp. n. (Diptera, Tabanidae) do Brasil. Acta Biol Par. 1997;26:23–32. https://doi.org/10.5380/abpr.v26i0.685.
-
Kruger RF, Krolow TK. Seasonal patterns of horse fly richness and abundance in the Pampa biome of southern Brazil. J Vet Ecol. 2015;40:364–72. https://doi.org/10.1111/jvec.12175.
-
Rodrigues GD, Blodorn E, Zafalon-Silva A, Domingues W, Marques R, Krolow TK, et al. Molecular detection of Trypanosoma kaiowa in Tabanus triangulum (Diptera: Tabanidae) from the coastal plain of Rio Grande do Sul, southern Brazil. Acta Parasitol. 2022;67:518–22. https://doi.org/10.1007/s11686-021-00440-1.
-
Reck C, Menin Á, Pisetta NL, Batista F, Miletti LC. First outbreak of autochthonous “surra” in horses in Santa Catarina State, Brazil: parasitological, hematological and biochemical characteristics. Vet Parasitol Reg Stud Rep. 2020;21:100427. https://doi.org/10.1016/j.vprsr.2020.100427.
-
Coscarón S, Papavero N. Manual of Neotropical Diptera. Tabanidae Neotrop Diptera. 2009;6:1–137.
-
Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen–Geiger climate classification. Hydrol Earth Syst Sci. 2007;11:1633–44. https://doi.org/10.5194/hess-11-1633-2007.
-
Christen S, Tavares KCS, Komati LKO, Ramos CJR, Miletti LC. SECTAB—a new device for tabanid storage in field collections. Neotrop Entomol. 2009;38:883–4. https://doi.org/10.1017/S1742758411000130.
-
Fairchild GB, Philip CB. A revision of the Neotropical genus Dichelacera subgenus Dichelacera, Macquart (Diptera, Tabanidae). Studia Entomol SP. 1960;3:1–86.
-
Claes F, Radwanska M, Urakawa T, Majiwa PA, Goddeeris B, Büscher P. Variable surface glycoprotein RoTat 1.2 PCR as a specific diagnostic tool for the detection of Trypanosoma evansi infections. Kinet Biol Dis. 2004;3:3. https://doi.org/10.1186/1475-9292-3-3.
-
Ventura RM, Paiva F, Silva RA, Takeda GF, Buck GA, Teixeira MM. Trypanosoma vivax: characterization of the spliced-leader gene of a Brazilian stock and species-specific detection by PCR amplification of an intergenic spacer sequence. Exp Parasitol. 2001;99:37–48. https://doi.org/10.1006/expr.2001.4641.
-
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–8.
-
Rodrigues A, Fighera RA, Souza TM, Schild AL, Soares MP, Milano J, et al. Outbreaks of trypanosomiasis in horses by Trypanosoma evansi in the state of Rio Grande do Sul, Brazil: epidemiological, clinical, hematological, and pathological aspects. Pesq Vet Bras. 2005;25:239–49.
-
Silva SS, Oliveira CB, Zanette RA, Soares CDM, Coradini G, Polenz CH, et al. Ocorrência de Trypanosoma evansi em bovinos de uma propriedade leiteira no município de Videira—SC, Brasil. Acta Sci Veter. 2007;35:373–376. https://doi.org/10.22456/1679-9216.16133
-
Zanette RA, Silva AS, Costa M, Monteiro SG, Santurio JM, Lopes STA. Ocorrência de Trypanosoma evansi em eqüinos no município de Cruz Alta, RS, Brasil. Ciência Rural. 2008;38:1468–71. https://doi.org/10.1590/S0103-84782008000500045.
-
Fermino BR, Paiva F, Viola LB, Rodrigues CMF, Garcia HA, Campaner M, et al. Shared species of crocodilian trypanosomes carried by tabanid flies in Africa and South America, including the description of a new species from caimans, Trypanosoma kaiowan sp. Parasit Vectors. 2019;12:2–17. https://doi.org/10.1186/s13071-019-3463-2.
-
Taioe MO, Motloang MY, Namangala B, Chota A, Molefe NI, Musinguzi SP, et al. Characterization of tabanid flies (Diptera: Tabanidae) in South Africa and Zambia and detection of protozoan parasites they are harboring. Parasitol. 2017;144:1162–78. https://doi.org/10.1017/S0031182017000440.
-
Werszko J, Szewczyk T, Steiner-Bogdaszewska Ż, Wróblewski P, Karbowiak G, Laskowski Z. Molecular detection of Megatrypanum trypanosomes in tabanid flies. Med Vet Entomol. 2020;34:69–73. https://doi.org/10.1111/mve.12409.
-
Bilheiro AB, Camargo JSAA, Zamaechi TBO, Tonholo C, Bassin HCM, Sussuarana ITA, et al. Survey of Trypanosoma (Kinetoplastida: Trypanosomatidae) infection in Monte Negro municipality, state of Rondônia, Western Amazon, with first record of T. evansi in the state. Rev Soc Bras Med Trop. 2019;52:e20190270. https://doi.org/10.1590/0037-8682-0270-2019.
-
Sumba AL, Mihok S, Oyieke FA. Mechanical transmission of Trypanosoma evansi and T. congolense by Stomoxys niger and S. taeniatus in a laboratory mouse model. Med Vet Entomol. 1998;12:417–22. https://doi.org/10.1046/j.1365-2915.1998.00131.x.
-
Desquesnes M, Biteau-Coroller F, Bouyer J, Dia ML, Foil L. Development of a mathematical model for mechanical transmission of trypanosomes and other pathogens of cattle transmitted by tabanids. Int J Parasitol. 2009;39:333–46. https://doi.org/10.1016/j.ijpara.2008.07.004.
Acknowledgements
The authors would like to thank Editage (www.editage.com) for the English language editing of an earlier draft of this manuscript and Dr. Inocêncio de Sousa Gorayeb for help with the taxonomy.
Funding
This study was supported in part by PAP UDESC—Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina grant no. 2017TR643 and Coordenação de Aperfeiçoamento de Nível Superior (CAPES).
Author information
Authors and Affiliations
Contributions
CJRR participated in the design of the study, the experiments, manuscript writing, and taxonomic identification. CF, SPL, LFNN contributed to sample collection. RSM, GBM, JM performed the experiments. LCM participated in the design of the study and manuscript writing. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
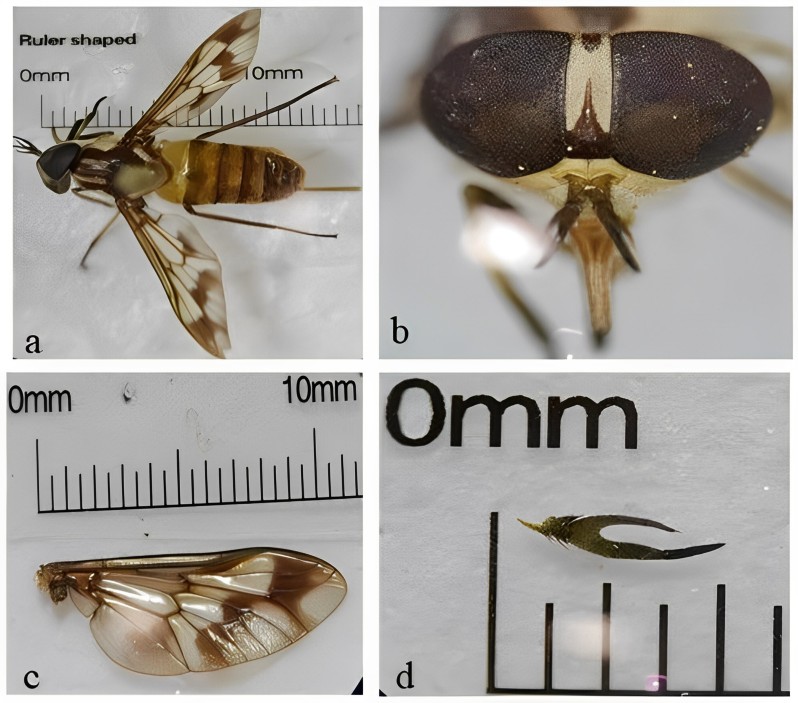
General morphology (a) and detail of head (b), wings (b), and antenna (d) of Dichelacera alcicornis. The scales are not the same in this composite figure.

General morphology (a) and detail of head (b), wings (c), and antenna (d) of Dichelacera januarii. The scales are not the same in this composite figure.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.














