A recent article in Nature indicated that scientists are at the cusp of developing a way to make gene-editing technology safer: by creating proteins which will allow a more targeted approach to gene manipulation. But what exactly is gene-editing in the first place, and why is a targeted approach necessary? The excitement behind this field grew exponentially in the last decade with the increasing popularity of CRISPR-Cas9, a biological complex found in bacteria. Essentially, bacteria in nature use this complex as a defense mechanism against invading viruses by targeting and disabling DNA sequences essential to viral functions. Scientists realized the potential application of this natural phenomenon: if this DNA splicing defense mechanism can be harnessed and expanded in scope, perhaps it can be used in humans to similarly target and disable disease-containing DNA sequences to eradicate the expression of the disease itself.
Accordingly, over the last decade, the scientific community has continued to streamline, expand upon, and bring forth practical and valuable applications in gene editing technology. A groundbreaking study in 2018 reported using CRISPR-Cas9 to edit specific gene-sequences in the Anopheles mosquito, a species that is commonly implicated with spreading malaria to humans. Using CRISPR-Cas9, scientists were able to inactivate a key gene in the mosquito that is essential to malaria growth; without this gene, the mosquitoes were unable to host, and therefore transmit the malaria parasite. The potential applications to this concept are monumental, from a global health perspective. With the World Health Organization (WHO) estimating that there are 400,000+ annual deaths due to malaria, a therapeutic model like this could be a significant benefit to public health initiatives globally.

Children sleep under long lasting insecticide nets (LLINs) that help repel bugs and prevent malaria.
Corbis via Getty Images
Additionally, the technology could change the face of clinical medicine. In 2017, it was reported that scientists used the Cas9 system to successfully curtail hearing loss in mice, a promising application in live animals for an ailment which has traditionally had few medical interventions available. Scientists have even expanded the scope into rare human blood disorders. Late last year, the preliminary findings on the first sickle-cell patient to use the CRISPR model as a curative therapy were released. Sickle cell disease entails a defect in mature hemoglobin which causes deformation (“sickling”) of red blood cells; these deformed red blood cells often stick to each other, causing severe episodes of pain (“pain crises”) in patients with the disease.
The CRISPR-Cas9 system was used to provide the test patient with stem-cells that could generate fetal hemoglobin, an alternative, more primitive form of hemoglobin that does not cause red blood cell deformation. Though still extremely early-on in the trial, initial results of the study (compiled by CRISPR Therapeutics, one of the firms that created the therapeutic model itself) indicated that the patient’s fetal hemoglobin level had increased to 46.6% just four months after the initial treatment was administered, compared to the 9.1% fetal hemoglobin the patient had prior to treatment. Additionally, one of the most significant aspects of this therapy was that the patient had not suffered from any pain crises since undergoing treatment. Both of these results indicate a promising improvement in quality of life for individuals with this disease. Ultimately, however, only time will truly tell whether this therapeutic model is indeed effective in the long-run and if there are any detrimental side effects, as many still don’t know what to fully expect from this treatment approach.
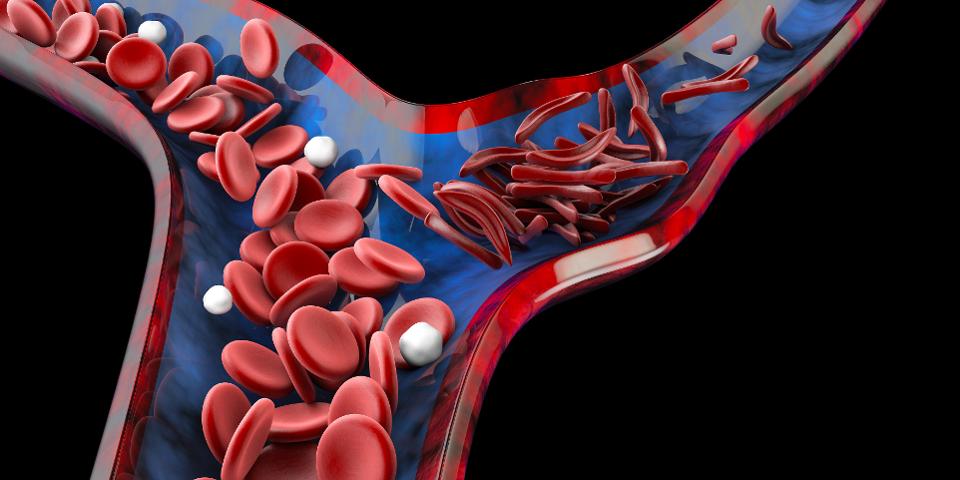
3D illustration of normal red blood cells (left) and deformed, “sickled” red blood cells (right).
Getty
While the conceptual idea and execution of this potential therapeutic model seem promising, ethical conundrums still exist with this technology. Namely, the sheer extent to which this intervention can be used is an overwhelming thought for many, especially given that the gene-editing model could potentially be used to do just as much genetic harm as it can benefit. Furthermore, how far should experimenting with this technology go, given its unmasked potential and unknown long-term effects? This latter issue was largely the topic of discussion when a Chinese scientist revealed in 2018 that he used this technology to genetically engineer the embryos that gave rise to two babies, a move that has since sparked international controversy and discussion on the ethical frameworks behind this therapeutic model.
Ultimately, this incident and the general ethical conundrums that have emerged indicate that significant questions remain open around gene-editing technology for the scientific, legal, political, and medical communities to answer. Indeed, this area of medicine will require strong ethical oversight, airing on the side of caution rather than on the side of experimentation and novelty. However, if these strict ethical standards are put into place and are duly respected, and this technology can be streamlined and perfected, gene-editing therapy potentially provides a promising outlook into the future of public health and disease management.