By Sarah Berry
Updated first published at
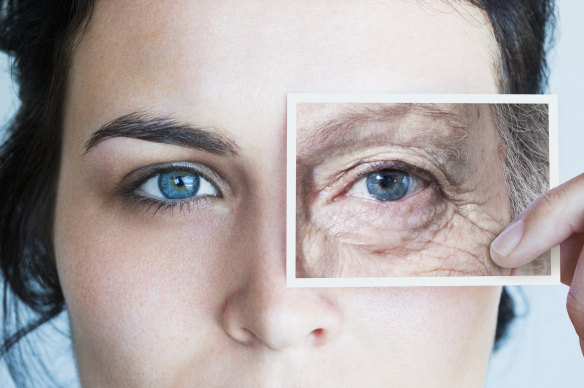
In the search for eternal youth, alchemists, magicians and scientists have tried it all. Now a new study has found that the cells in our bodies can be rebooted and the ageing process can potentially be reversed.
It was once believed that ageing was the result of DNA damage that we accumulate over our lifetimes, and that this damage is accelerated by smoking, exposure to pollution, too much sun and poor diet.

Can we turn back the clock of ageing? Scientists are trying to.Credit:Getty
Then, in 1962, a British scientist, John Gurdon did a series of experiments with frogs in which he took out DNA from a frog’s egg and replaced it with DNA from a mature cell. If the theory about ageing and accumulated DNA damage was true, placing mature DNA in an egg would produce an old frog. But it didn’t. The hardware of the aged cell didn’t appear to be the problem. The frogs that hatched were perfectly normal and healthy.
It suggested that there was something floating around inside the egg cell which had the ability to reprogram that old DNA – update its software – and make it young again.
Figuring out what that something was would take another 50 years. In 2006, Japanese researcher Shinya Yamanaka discovered the four genes (OSKM) – collectively known as Yamanaka factors – that could clear the slate of ageing in a mature cell, returning it to its embryonic state.
Loading
Since then, researchers tried reversing the ageing process in animals via reprogramming the gene expression – the way genes are switched on or off and how they behave (this process is known as epigenetics) – but it was fraught.
“People have tried to reprogram within the context of a whole animal and that leads to the risk of what are called teratomas,” explains Dr Lindsay Wu, a senior research fellow who leads Laboratory for Ageing Research at The University of New South Wales.
Teratomas are a type of tumour that are made of the wrong tissue, including teeth, hair, bone and muscle, in the wrong part of the body.
“That’s why we haven’t really seen that field advance since the mid-to-late two thousands.”
For more than a decade, Australian biologist David Sinclair, a professor of genetics and co-director of the Paul F. Glenn Center for Biology of Aging Research at Harvard Medical School, has been a leading voice in anti-ageing research and lifespan extension.
Instead of trying to create a blank slate in the cells, Sinclair (who considers ageing a “disease”) has focused on partially resetting the epigenetic instructions.
This is because he believed the hypothesis that the more damaged the DNA, the more disrupted the epigenetic signals would become, leading to cellular dysfunction and senescence (when a cell ages and permanently stops dividing but does not die). In other words, it was epigenetics, not just DNA damage driving ageing.
He wondered whether ageing was essentially a “glitch in the software of the body” that causes it to malfunction. By rebooting the software, restoring the cell’s ability to read its original DNA so it remembers how to function, he hoped he could fix the glitch.
In a paper published in Nature in 2020, Sinclair and his team were able to restore vision to old mice by injecting their eyes with a benign virus carrying three of the four Yamanaka factors (OSK).
Loading
In his new paper, published this week in Cell, he took this research a step further, partially turning back the biological clocks in multiple organs, including the eyes, of mice they prematurely aged.
“We think of the processes behind ageing, and diseases related to ageing, as irreversible,” Sinclair told TIME. “In the case of the eye, there is the misconception that you need to regrow new nerves. But in some cases, the existing cells are just not functioning, so if you reboot them, they are fine. It’s a new way to think about medicine.”
The 53-year-old added: “Underlying ageing is information that is lost in cells, not just the accumulation of damage. That’s a paradigm shift in how to think about ageing.”
Dr Christian Nefzger the group leader in the Cellular Reprogramming and Ageing laboratory at the University of Queensland says: “It should be considered a seminal study as it most directly links epigenetic erosion to ageing.”
But, it’s still very early days. “Conceptually, the risk is that you could induce too much reprogramming,” says Wu, who has worked with Sinclair and is involved in a company with him. “That’s why there is obviously a lot of caution being used here.”
Nefzger agrees. Although he says it is good news as epigenetic changes can potentially be reversed, unlike DNA mutations, he adds: “There are safety and efficacy concerns regarding the gene therapy approach used here, and it should not be considered a viable treatment option as of now.”
Along with the risk of teratomas, such a treatment “might induce cancer in longer-lived species like ours”. We also do not yet understand what other, potentially undesirable epigenetic changes might be introduced with this kind of tinkering.
Though it holds promise as a targeted therapy for certain age-related diseases, Nefzger anticipates it will take decades more research to get it right and ensure its safety.
The next step for Sinclair and his team is to test the approach in non-human primates. They are also continuing to experiment with switching on and off cells throughout the body to try and reverse ageing entirely.
Of this, Wu says:
“We’ve already got interventions which are much safer and much more powerful in terms of their ability to keep us young and healthy.”
Those interventions are quality diet and exercise, maintaining muscle mass via resistance training, eating less, avoiding sun damage and getting good sleep. Sinclair’s own anti-ageing regime involves regular exercise, sauna steams and ice baths, only one or two meals a day, a mostly vegetarian diet and taking the diabetes drug metformin as well as a range of supplements.
Wu says that in our lifetime we are likely to see these therapies rolled out in humans to treat specific issues, but we are unlikely to see them being used in humans to treat ageing itself.
“Testing whether an intervention makes you younger or extends the overall life span in humans is really difficult to do,” he says. “It would take a very long time.”
Make the most of your health, relationships, fitness and nutrition with our Live Well newsletter. Get it in your inbox every Monday.
Sarah Berry is a lifestyle and health writer at The Sydney Morning Herald and The Age.Connect via Twitter or email.
Loading